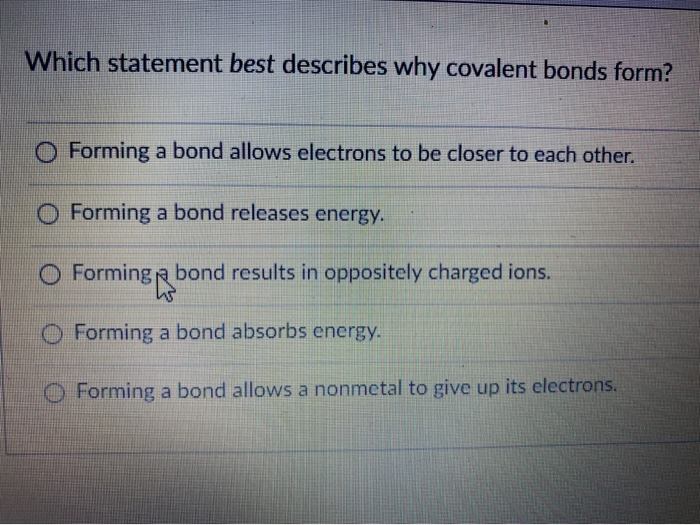
Which Statement Best Describes Why Covalent Bonds Form
Forming a bond allows a nonmetal to give up its electrons. Which statement best describes why covalent bonds form.

Ionic Versus Covalent Bonds Chemical Formula And Chemical Naming Chemistry Lessons Covalent Bonding Chemistry Notes
2 Which statement best explains why carbon is present in so many kinds of molecules it can form four covalent bonds.

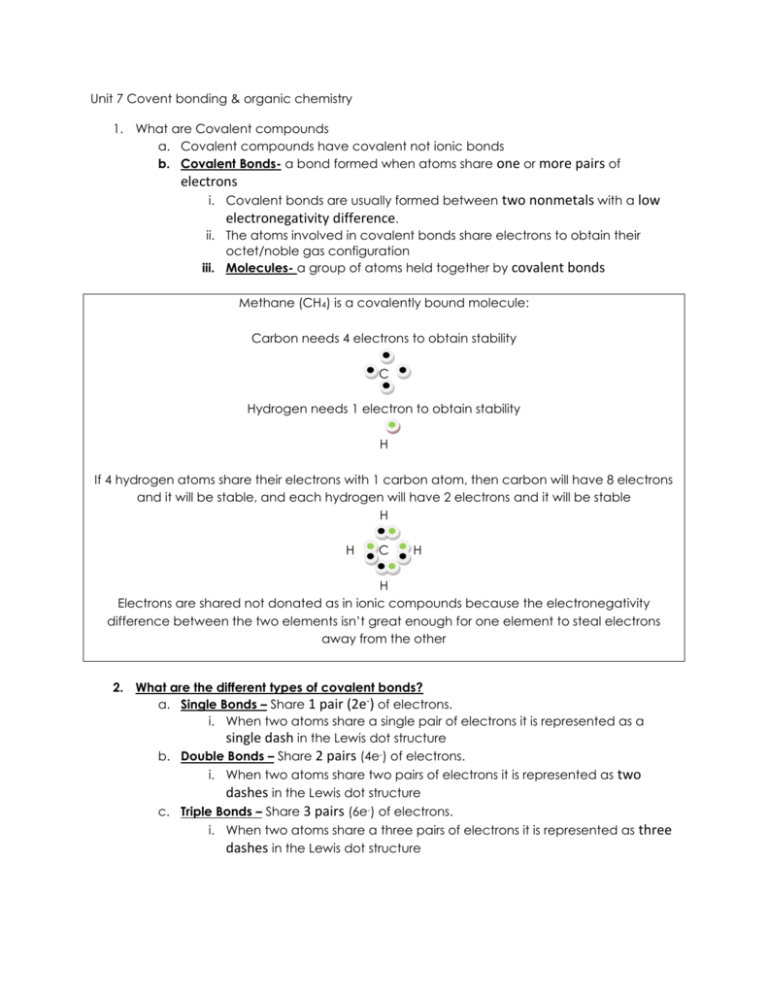
. Lectronegativity is the ability of an atom to attract toward itself the electrons in a chemical bond. A chemical bond that involves sharing a pair of electrons between atoms in a molecule. The electrons involved are located in the atoms outer shells.
Indicate whether you agree or disagree with the students plan and why. The outer shell of an atom would be completed if a or more share electrons. Which statement correctly describes a covalent bond.
The hydrogen bond is now stable since it is in contact with two electrons in its outer shell. 2 Questions Show answers. Which Statement Best Describes How An Ionic Bond Forms.
The atoms valence electrons combine to form a network of bonds. For example diamond is a covalent network solid in which carbon atoms are arranged in a continuous lattice like structure. The best reason for why a covalent bond forms is atoms with high electronegativities will not lose electrons so instead they share electrons to reach a stable number of valence electrons.
4 Which reason best explains why carbon is able to form macromolecules quizlet. Which statement best explains why atoms form chemical bonds with other atoms. A metal and nonmetal react to form an ionic bond.
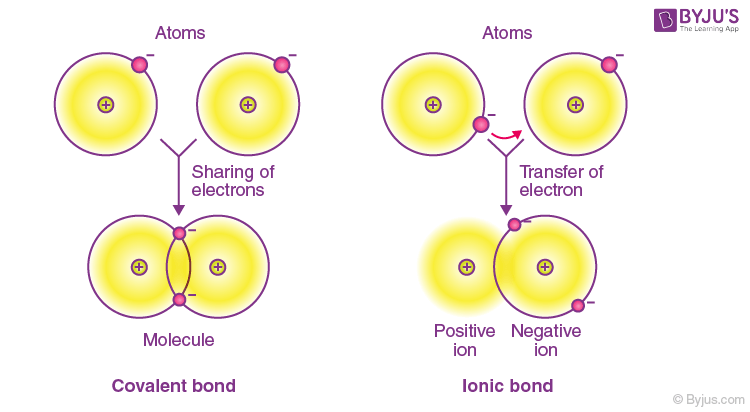
Consider a water molecule. Covalent bonds would most likely form between. Valence electrons are transferred from one atom to the other.
Polar covalent bond or polar bond is a covalent bond with greater electron density around one of the two atoms. What statement best describes the actions of electrons in an ionic bond. Forming a bond releases energy.
It has a negative charge that is spread over the entire ion. Both statements are TRUE B. H--O--H each dash represents a covalent bond.
3 Which characteristic best explains why carbon is important to living organisms. When an atoms with a strong attraction for electrons ON bonds with atoms with a low affinity for electrons CH the electrons are not shared equally and result in a polar covalent bond. By sharing their outer most valence electrons atoms can fill up their outer electron shell and gain stability.
Which of these statements best describes a dative covalent bond. When two non-metal atoms share a pair of electrons a covalent bond is formed. Forming a bond results in oppositely charged ions.
Forming a bond releases energy. Forming a bond results in oppositely charged ions. They dont share electrons.
Which statement best describes a covalent bond. An _____ is the smallest particle of a covalent compound that still has the properties of the compound. Forming a bond allows a nonmetal to give up its electrons.
Alone each oxygen atom has six valence electrons. Its outermost shell gains one or more electrons from Cl-. Covalent bonds can form between two nonmetal atoms.
Forming a bond absorbs energy. O Forming a bond absorbs energy. A pair of electrons shared between two atoms where each atom has donated one electron.
Therefore the final answer is Option B. Bonds form because of opposite charges. Ionic bonds are like covalent bonds because both type of bonds _____.
Formingp bond results in oppositely charged ions. D Most atoms are unstable unless they are combined with other atoms. The student plans to break the copper into three pieces by bending it.
A metalloid and oxygen. Which statement best describes why covalent bonds form. The atoms valence electrons are shared between the atoms.
Forming a bond allows electrons to be closer to each other. O Forming a bond allows electrons to be closer to each other. Forming a bond absorbs energy.
Forming a bond allows electrons to be closer to each other. Covalent bonds are magical. Covalent Bonds do share electrons.
Look at the oxygen atoms in the Figure above. Hence we can conclude that the statement all the atoms are covalently bonded to other atoms to form a lattice-like structure best describes the structure of covalent network solids. A student is given a solid piece of copper wire and is asked to perform three separate experiments using the sample of the metal.
Covalent bonds form because they give atoms a more stable arrangement of electrons. An ionic bond is a type of chemical bond formed through an electrostatic attraction between two oppositely charged ions. O Forming a bond allows a nonmetal to give up its electrons.
Which statement best describes why covalent bonds form. An ionic bond is most likely to form between. Which best describes why NH4 can form an ionic bond with Cl-.
By sharing two pairs of valence electrons each oxygen atom has a total of eight valence electrons. Atoms will covalently bond with other atoms in order to gain more stability which is gained by forming a full electron shell. 5 Which statement best explains why a carbon atom can bond easily.
O Forming a bond allows a nonmetal. All of the atoms electrons are shared between the atoms. Bonds form to fill outer electron shells.
Electrons are transferred between atoms. Carbon forms covalent bonds because carbon has an atomic number 6 and have 4 electrons in its octet so it can neither lose nor gain 4 electrons to complete its octet so it forms covalent bonds by sharing its 4 electrons and covalent bonds. Its positive charge is attracted to the negative charge of Cl-.
The attraction between 2 metal atoms. A pair of electrons shared between two atoms where one atom has donated two electrons. Ionic bonds form when a nonmetal and a metal exchange electrons while covalent bonds form when electrons are shared between two nonmetals.
O Forming a bond releases energy. Covalent bonding occurs when pairs of electrons are shared by atoms. Chemistry questions and answers.

Covalent Bond Definition Types And Examples

Covalent Bonds Flashcards Quizlet
Solved Which Statement Best Describes Why Covalent Bonds Chegg Com

Single And Multiple Covalent Bonds Article Khan Academy

Single And Multiple Covalent Bonds Article Khan Academy

Bonding Covalent And Ionic Bonds Shmoop Chemistry Covalent Bonding Ionic Bonding Teaching Chemistry

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond

Ionic Bond Vs Covalent Bond Venn Diagram Shows The Similarities And Differences Between The Chemical Bonds Click Covalent Bonding Ionic Bonding Chemical Bond

Covalent Bond Definition Properties Examples Facts Britannica

Covalent Bond Definition Types And Examples

Which One Of The Following Best Describes A Job Cost Sheet In 2022 Cost Sheet Job Sheet
How Do Covalent Bonds Form In The New Model Where The Electron Is In A Probability Cloud Quora

Lesson 37 Covalent Compounds Objectives The Student Will Define Covalent Bond And Molecular Compound The Student Will Classify Bonds As Ionic Polar Ppt Download

Covalent Bond Definition Types And Examples

Help Students Understand How To Draw Lewis Structures Understand Molecular Geometry And The Polar Nature Of Ion Molecular Shapes Molecular Geometry Molecular


Comments
Post a Comment